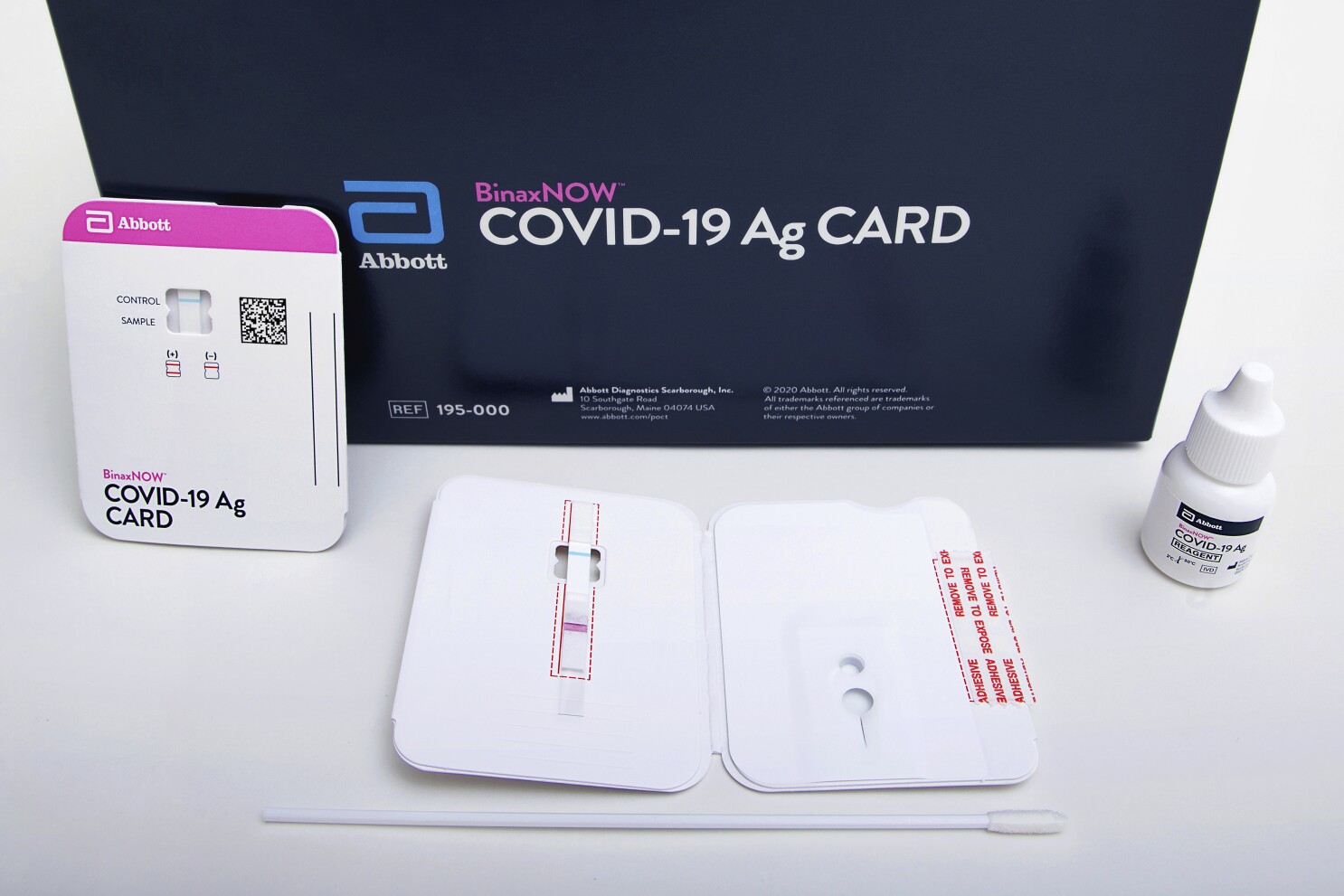
DIGITAL POINT OF CARE PLATFORM PROVIDING VERIFIED TESTING AND ACCESS TO RX TREATMENT. The 15-minute BinaxNOW COVID-19 Ag Card a rapid antigen test was designed alongside the revolutionary NAVICA digital system for testing in CLIA-waived settings like universities offices pharmacies long term care facilities.

Abbott Agrees With Fda To Perform Post Market Study On Quest
Abbotts tests run on its Alinity automated molecular diagnostics analyzer.

. Taking COVID-19 testing programs to a new level. Find out more about our best-in-class products and solutions. The terms and conditions of coverage for at-home COVID-19 testing vary by insurance plan so.
Food and Drug Administration Emergency. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. All are available in the US.
What requirements exist for those receiving the test kits. Department of Health and Human Services HHS has begun distribution of Abbott BinaxNOW COVID-19 Antigen Ag Card Point of Care POC SARS-CoV-2 diagnostic tests. Recipients include states long term care facilities LTCF and historically black colleges and universities HBCUs.
Curative is among the companies to adopt the. Designed to restore confidence in daily life. CEO Robert Ford picked out the rollout of the analyzer as a driver of growth in Abbotts underlying diagnostics business in July.
BinaxNOW COVID-19 Ag Card has received US. Ford previously said launching the platform with the COVID-19 test helped jumpstart demand. NAVICA displays results from the 15-minute Abbott BinaxNOW COVID-19 Ag Card rapid antigen test to help individuals make informed decisions.
BinaxNOW COVID-19 Ag Card joins our previously launched tests including high-volume m2000 and Alinity m molecular laboratory systems ID NOW rapid molecular point-of-care platforms antibody tests for our high-throughput ARCHITECT i1000SR and i2000SR and Alinity i laboratory instruments. Due to a federal government mandate eMeds at-home COVID-19 test may likely be covered by your insurance. Abbott Rapid Diagnostics formally Alere and Point of Care Testing POCT.

Abbott Id Now Covid 19 Detection Test System Us
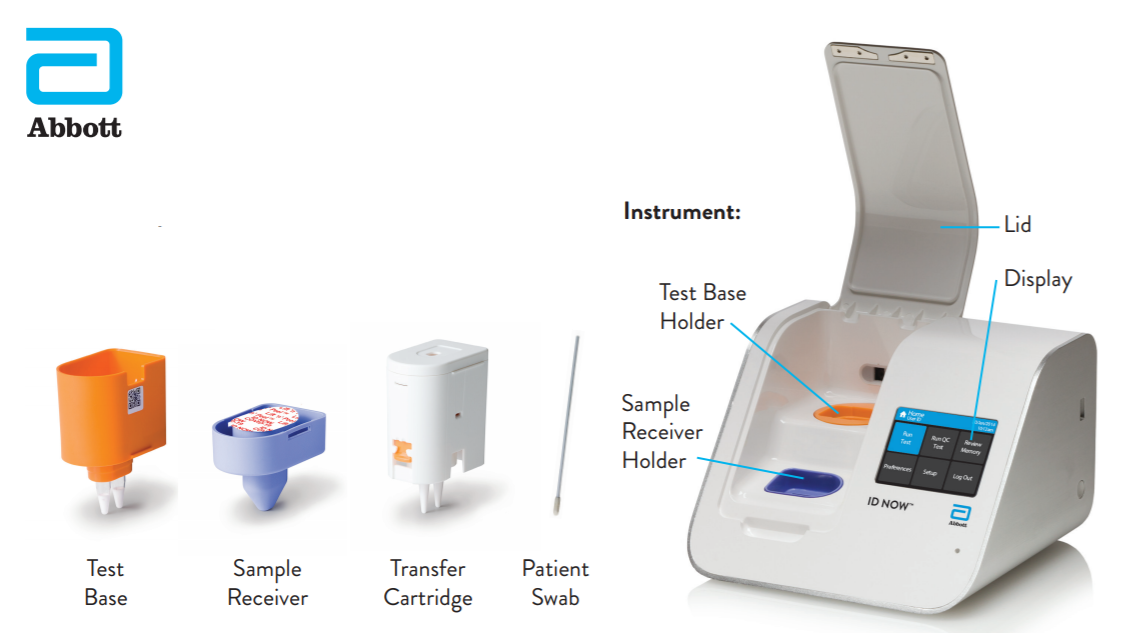
Instant Results From Abbott 39 S Covid 19 Point Of Care Tes
Panbio Covid 19 Ag Test Abbott Point Of Care
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/66569043/IDNOW_INACTION3_macro_300dpi_1200x628.0.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Id Now Covid 19 Abbott Point Of Care
Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S

